Gas That Exerts the Greater Partial Pressure
Show the complete solution a. Partial pressure P x is the pressure of a single type of gas in a mixture of gases.
Solved A Flask At Room Temperature Contains Exactly Equal Chegg Com
Equal way are equal Pressure is a big fat which Mullen for after off again outing the Greater Africa velocity.
. For example in the atmosphere oxygen exerts a partial pressure and nitrogen exerts another partial pressure independent of the partial pressure of oxygen Figure 2241. Which of the two gases exerts the greater partial pressure. Since every gas has an independent behavior the ideal gas law is used to find the.
The molecules or atoms of which gas have the greater average velocity. Partial pressure is the pressure that an individual gas exerts in a mixture of gases which in distillation can have an effect on boiling so pressure may have to be increased to achieve the boiling temperature. Gas that exerts the greater partial pressure.
Between these Oh no. B The helium gas exerts about four times as much pressure as the hydrogen gas. According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual gas and every gas is assumed to be an Ideal gas.
Yeah It passed the moon you know. The molecules of which gas will have the greater average kinetic energy. N P V RT 969 atm1730 cm3 82054 cm3 K1 mole1 298 K n P V R T 969 atm 1730 cm 3 82054 cm 3 K 1 mole 1 298 K n 0068 moles O 2.
What was the original volume. The mole fraction of oxygen gas in the bottle is 0969 not 1000 and the partial pressure of oxygen also is 0969 atm. Fluorine gas exerts a pressure of 9065 torr.
The partial pressure of carbon dioxide is also different between the alveolar air and the blood of the capillary. What is the partial pressure of ethane Pethane in the flask. The valve is opened and the two gases equili-brate.
Gas that exerts the greater partial pressure. Where P 1 P 2 P 3 are the partial pressures of gas 1 gas 2 and gas 3. Gas for which the molecules or atoms have the greatest average velocity.
A gas at constant temperature occupies a volume of 559 L and exerts a pressure of 883 torr. Pethane 0615 atm Daltons law of partial pressures states that the total pressure of a system is equal to the sum of the partial pressures of the component gases in the mixture. Gas for which the molecules or atoms have the greatest average velocity C.
Gas for which the molecules or atoms have the greatest average kinetic energy. One ofthe nitro again. Plus more lots in there Fast Jack We dont need to have any practical you know Does one ofthe night.
Which gas exerts the greater partial pressure and by what factor. We see from eq3 above that a gas mixture that obeys Daltons law of partial pressures obeys the ideal gas law with the total number of moles n total. V A 1 L V B 1 L P A 811 torr P B 277 torr V total 2 L P AV A P tV t P t P AV A V t.
Which of the two gases exerts the greater partial pressure. Choose the best answer. The average kinetic energy of a collection of gas particles is assumed to be proportional to the Celsius temperature of the gas 8.
P total P 1 P 2 P 3. The expression for Daltons law of partial pressure is PtotalPAPB. Gas that would have a higher rate of effusion through a small hole opened in the flask.
Chlorine exerts the highest partial pressure at a certain temperature. Nitrogen has the greater value 2. A Nitrogen b Xenon c They will exert the same partial pressure.
The number of moles is. The molecules of which gas have. The partial pressure of a single gas is proportional to the percentage of the gas in a mixture of gases.
So there we are. Greater the mole fraction of a gas in gaseous mixture greater is the pressure it exerts. The molecules or atoms of which gas will have the greater average velocity.
Nitrogen has the greater value Xenon has the greater value. A The helium gas exerts about twice as much pressure as the hydrogen gas. Which of the two gases exerts the greater partial pressure.
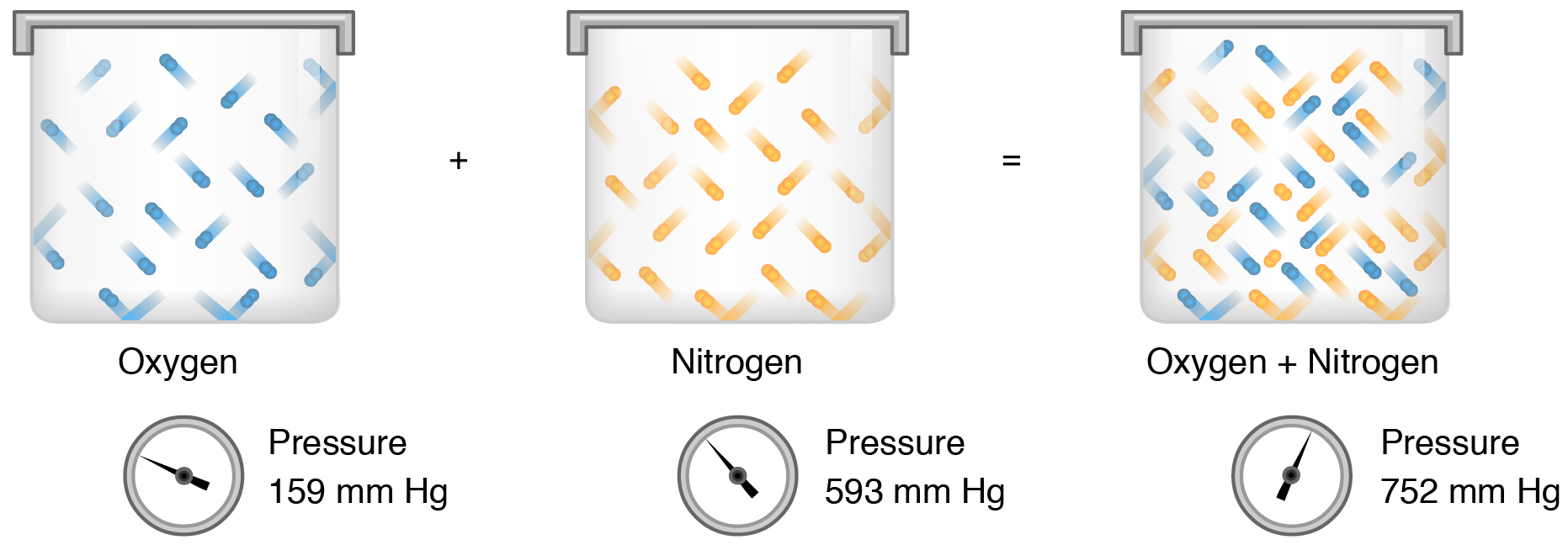
Total pressure is the sum of all the partial pressures of a gaseous mixture. For example in the atmosphere oxygen exerts a partial pressure and nitrogen exerts another partial pressure independent of the partial pressure of oxygen Figure 2221. Reason Pressure of gas is due to bombardment of gas molecules on the walls of the container.
Daltons law of partial pressures states that the total pressure of a mixture of gases equals the sum of the partial pressures of the component gases that is the ΣP i P total. In any mixture of gases each component gas exerts a partial pressure that contributes to the total pressureAt ordinary temperatures and pressure you can apply the ideal gas law to calculate the partial pressure of each gas. Gas that exerts the greater partial pressure.
A flask at room temperature contains exactly equal amounts in moles of nitrogen and xenon. Gas B ex-erts a pressure of 277 torr in a 1 liter bulb. Partial pressure P x is the pressure of a single type of gas in a mixture of gases.
Nitrogen has the greater value 3. Gas for which the molecules or atoms have the greatest average velocity. When the pressure is changed to 150atm its volume is 2520mL.
The molecules of which gas have the greater average kinetic energy. Consider a mixture of equal masses of helium and hydrogen gases in a steel container. Gas for which the molecules or atoms have the greatest average of kinetic energy.
What is the partial pressure of gas A expressed after equilibration. Daltons law of partial pressure states that the pressure exerted a mixture of gases is equal to the sum of the partial pressure of each individual gas. Gas that would have a higher rate of effusion through a small hole opened in the flask.
Chlorine gas exerts a partial pressure of. However the partial pressure difference is less than that of oxygen about 5 mm Hg. Bulb at a pressure of 811 torr.
Both gases have the same value 4. Gas that would have a higher rate of effusion through a small hole opened in the flask. Total pressure is the sum of all the partial pressures of a gaseous mixture.
The partial pressure of carbon dioxide in the blood of the capillary is about 45 mm Hg whereas its partial pressure in the alveoli is about 40 mm Hg. If a small hole were opened in the flask which gas would effuse more quickly. 3 Both gases have the same value A.


Comments
Post a Comment